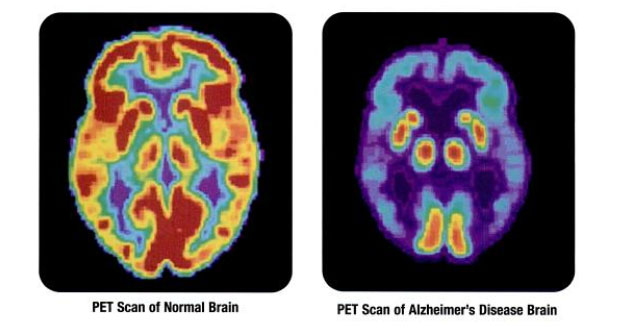
Clinical stage biopharmaceutical company Cortexyme Inc. (CRTX:NASDAQ), which is focused on developing disease-modifying therapeutics to treat Alzheimer's and other degenerative diseases, today announced financial results for its second quarter ended June 30, 2021 and provided an update on expected clinical topline data from its pivotal Alzheimer's disease trial.
Cortexyme's CEO, Cofounder and Chair Casey Lynch commented, "Evidence demonstrating a causal link between the infectious pathogen P. gingivalis and neurodegeneration continues to grow. We are pleased with the rigorous and efficient execution of the GAIN trial and are excited to be rapidly approaching such a significant milestone for our industry."
"We have developed a potential therapeutic with a breakthrough mechanism of action upstream of multiple aspects of Alzheimer's disease pathology including inflammation and neurodegeneration, and we look forward to sharing a robust topline data set by mid-November 2021," Lynch added.
The company's Chief Medical Officer Michael Detke, M.D., Ph.D. remarked, "We believe positive data from the GAIN trial could fundamentally shift the paradigm of neurodegeneration research and disease-modifying treatment in the Alzheimer's field and we intend to rapidly pursue collaboration with the FDA for the benefit of patients."
Cortexyme advised that its pivotal Phase 2/3 GAIN trial enrolled 643 patients diagnosed with mild to moderate Alzheimer's disease and mentioned that it expects to receive topline data from the study in mid-November 2021. The company claimed that it is pioneering a novel upstream, disease-modifying therapeutic approach to treating Alzheimer's disease.
The company explained that the GAIN trial is powered at approximately 90% and is designed with the goal of delivering a 50% slowing in the decline of cognition in Alzheimer's patients measured by co-primary clinical endpoints of ADAS-Cog11 and ADCS-ADL.
The firm stated that concurrently it is conducting its Phase 2 REPAIR sub-study of 233 patients addressing periodontal disease. Cortexyme said that at present fewer than 100 individuals are pending treatment completion in the study. Cortexyme advised that the Phase 2 periodontal disease REPAIR trial is a substudy of the GAIN trial aimed at evaluating standard clinical endpoints of periodontitis, such as bleeding on probing, clinical attachment level and gingival pocket depth.
Cortexyme stated that "it presented new data at the International Association for Dental Research (IADR) 2021 demonstrating atuzaginstat disrupts biofilms and is efficacious in preclinical models of periodontal disease." The company reported that atuzaginstat does this by penetrating and disrupting bacterial biofilms, which is a key facet of P. gingivalis treatment. The firm additionally pointed out that administration of atuzaginstat at dosage levels similar to those used to treat periodontal disease has been shown to reverse alveolar bone loss in mice after oral P. gingivalis infection.
The company did not report any revenues in the most recent quarter and advised that in Q2/21 it posted a net loss of $21.8 million, or $0.74 per basic share. The firm indicated that the weighted average shares outstanding during the period were 29,587,352.
Cortexyme noted that as of June 30, 2021, it held $153.5 million in cash, cash equivalents and short and long-term marketable securities on its balance sheet, which it anticipates will be sufficient to fund the company's operations until the end of 2023.
The company listed that it spent a total $14.7 million on research and development (R&D) expenses in Q2/21. The firm noted that the majority of the expenses incurred were associated with R&D efforts involving atuzaginstat, the GAIN trial, COR588 and other related research staffing related expenses. The company added that in Q2/21 it spent $7.1 million on general and administrative expenses primarily for salaries, insurance and various legal and professional fees.
Cortexyme is a clinical-stage biopharmaceutical company headquartered in South San Francisco, Calif. The firm engaged in pioneering a new upstream disease-modifying therapeutic approach to treat the key underlying causes of Alzheimer's and other degenerative diseases. The company's research is focused on targeting a specific, infectious pathogen found in the brain of Alzheimer's patients called P. gingivalis that has previously been tied to neurodegeneration and neuroinflammation both in humans and in animal studies. Along with its ongoing GAIN study centered around treatments for mild to moderate Alzheimer's disease, the company also has a proprietary research pipeline geared toward developing first-in-class small molecule therapeutics for Parkinson's disease, periodontitis and other diseases.
Cortexyme started the day with a market cap of around $2.0 billion with approximately 29.59 million shares outstanding and a short interest of about 9.7%. CRTX shares opened slightly lower today at $66.62 (-$0.52, -0.77%) from Friday's $67.14 closing price and then turned higher reaching a new 52-week high price this afternoon of $99.99. The stock has traded today between $66.23 to $99.99 per share and closed at $98.91 (+$31.77, +47.32%).
[NLINSERT]
Disclosure:
1) Stephen Hytha compiled this article for Streetwise Reports LLC and provides services to Streetwise Reports as an independent contractor. He or members of his household own securities of the following companies mentioned in the article: None. He or members of his household are paid by the following companies mentioned in this article: None.
2) The following companies mentioned in this article are billboard sponsors of Streetwise Reports: None. Click here for important disclosures about sponsor fees.
3) Comments and opinions expressed are those of the specific experts and not of Streetwise Reports or its officers. The information provided above is for informational purposes only and is not a recommendation to buy or sell any security.
4) The article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports' terms of use and full legal disclaimer. This article is not a solicitation for investment. Streetwise Reports does not render general or specific investment advice and the information on Streetwise Reports should not be considered a recommendation to buy or sell any security. Streetwise Reports does not endorse or recommend the business, products, services or securities of any company mentioned on Streetwise Reports.
5) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned. Directors, officers, employees or members of their immediate families are prohibited from making purchases and/or sales of those securities in the open market or otherwise from the time of the decision to publish an article until three business days after the publication of the article. The foregoing prohibition does not apply to articles that in substance only restate previously published company releases.
6) This article does not constitute medical advice. Officers, employees and contributors to Streetwise Reports are not licensed medical professionals. Readers should always contact their healthcare professionals for medical advice.