PolarityTE Inc. (COOL:NASDAQ) has developed a tissue engineering (TE) platform that uses the patient's healthy tissue, as opposed to using synthetic or foreign materials inside an artificially engineered environment.
The company's first commercially launched product is SkinTE, which uses a small amount of a patient's own skin to regenerate damaged skin.
Cantor Fitzgerald initiated coverage of PolarityTE on Feb. 14 with a $70/share target price, more than triple the current share price $21.22.
"We believe that PolarityTE can revolutionize the field of regenerative medicine; our target price is $70." – Elemer Piros, Cantor Fitzgerald
Elemer Piros Ph.D., an analyst with Cantor Fitzgerald, wrote, "We believe that PolarityTE can revolutionize the field of regenerative medicine. The company achieved previously unseen results with its SkinTE autologous cell product in wound healing, demonstrating flawless healing in animals and emerging evidence from the first human patients."
He noted that "the team at PolarityTE deciphered a technique, involving minimal manipulation of the patient's own healthy skin, to expand to produce fully functional skin 500-1,000 times the original size, with minimal/to no scarring. A dime-sized biopsy can yield tissue to cover an entire arm."
"PolarityTE began to build out a 200,000 square feet campus (size of an airplane hangar housing 2-3 747s), which can process ~230,000 products/year," Piros stated.
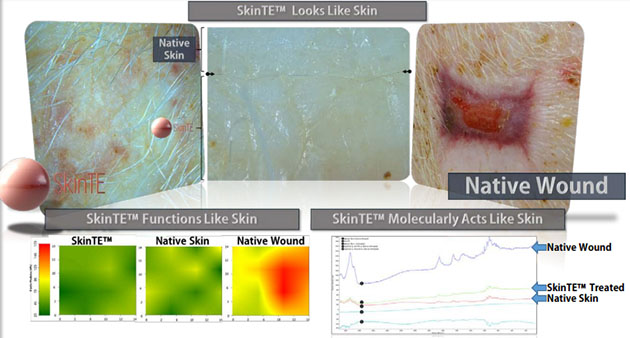
In the preclinical research, applications of SkinTE resulted in "regenerative healing of full-thickness hair-bearing skin, including all layers (epidermis, dermis, and hypodermis) and glands." This compares to animal-based tissues (xenografts) or cadaveric-based tissues (allografts) that "don't result in actual tissue regeneration."
The company has stated that the initial results on patients are "yielding outcomes that are correlative with the Company's preclinical research."
A physician sends to PolarityTE's facility a one-centimeter piece of a patient's skin, which is then engineered and sent back to the physician, who applies it to the wound.
"If SkinTE becomes widely adopted and used in the medical world as it looks likely, we could see explosive growth in this stock." – Clive Maund
The patient's body, according to the company "then provides a receptive environment and nutrients for controlled healing. The body uses its own natural biological healing process, which SkinTE believes will support the regenerative process. Not only does the company believe the tissue can be regenerated but its "natural coloring, texture, layers and structure, hair and appendages" are included too.
The engineered skin has been shown to heal at a much faster rate than traditional therapies.
"We manufacture with the patient's own tissue and use the patient's own body to support the regenerative process to create the same tissue from which it was derived. We believe that our innovative method promotes and accelerates growth of the patient's tissues to undergo a form of effective regenerative healing," PolarityTE stated.
A perk with using the patient's own healthy tissue is that it increases the chances that the patient's immune system will identify the regenerating tissue as its own so that SkinTE is neither rejected nor reacted to adversely, PolarityTE argues.
SkinTE is registered with the FDA "pursuant to the regulatory pathway for human cells, tissues, and cellular and tissue-based products (HCT/Ps) regulated solely under Section 361 of the Public Health Service Act, or 361 HCT/Ps, which permits qualifying products to be marketed without first obtaining FDA marketing authorization or approval."
Since November 2017, the company says, it has sold and provided SkinTE to multiple medical providers across the country who treated patients for acute and chronic wounds, surgical reconstruction, burns, and removal of scarred and contracted skin grafts for replacement with SkinTE.
Gerhard S. Mundinger, MD, Director of Plastic Surgery at Children’s Hospital in New Orleans, Assistant Clinical Professor of Plastic Surgery, and Assistant Professor of Cell Biology and Anatomy at LSU Health New Orleans, stated "I have used SkinTE to partially resurface extensive burn scars and skin graft contractures in an adolescent patient. Although early in the post-operative period, the product has quickly regenerated skin with minimal marginal wound contracture and areas of regenerated skin are re-pigmenting rapidly."
Denver Lough MD, PhD, PolarityTE's CEO, says, "We at PolarityTE are incredibly excited to see how SkinTE, the first product from our core tissue 'TE' technology platform, is beginning to impact the lives of patients through the regeneration of their own complete skin."
PolarityTE believes that its platform "has the potential to transform tissue regeneration, including potential regeneration of multiple tissue substrates, such as skin, bone, muscle, fat, cartilage, nerves, and blood vessels."
PolarityTE expects to register its bone regeneration product, OsteoTE, with the FDA and start selling it commercially by the end of 2018.
The company also mentioned other regenerative products in the works: CartTE (cartilage for osteoarthritis, facial reconstruction, and more), AdipoTE (fat transfers for plastic surgery procedures), AngioTE (vascular grafts), NeuralTE (nerve repair), UroTE (urogenital epithelium and submucosa), LiverTE (liver tissue for liver fibrosis or failure), and BowelTE (bowel tissue).
Technical analyst Clive Maund on Feb. 13 wrote on CliveMaund.com that "This small company, with a relatively tiny float of stock, has developed a much more advanced method of treating serious burns that is very fast, works, and is likely to be taken up and adopted by hospitals and burns units across the country and even around the world. The upside potential of this stock is therefore big."
"Keep in mind," Maund wrote, "the very low float of this stock. . ., which makes very big percentage gains possible, and that, if the company’s principal product, SkinTE, becomes widely adopted and used in the medical world as look likely, we could see explosive growth in this stock."
Want to read more Life Sciences Report articles like this? Sign up for our free e-newsletter, and you'll learn when new articles have been published. To see recent articles and interviews with industry analysts and commentators, visit our Streetwise Interviews page.
Disclosure:
1) Nikia Wade and Patrice Fusillo compiled this article for Streetwise Reports LLC and provide services to Streetwise reports as an independent contractor and as an employee, respectively. They or members of their households own securities of the following companies mentioned in the article: None. They or members of their households are paid by the following companies mentioned in this article: None.
2) The following company mentioned in this article is a billboard sponsor of Streetwise Reports: None. Click here for important disclosures about sponsor fees.
3) Comments and opinions expressed are those of the specific experts and not of Streetwise Reports or its officers. The information provided above is for informational purposes only and is not a recommendation to buy or sell any security.
4) The article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports' terms of use and full legal disclaimer. This article is not a solicitation for investment. Streetwise Reports does not render general or specific investment advice and the information on Streetwise Reports should not be considered a recommendation to buy or sell any security. Streetwise Reports does not endorse or recommend the business, products, services or securities of any company mentioned on Streetwise Reports.
5) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned. Directors, officers, employees or members of their immediate families are prohibited from making purchases and/or sales of those securities in the open market or otherwise from the time of the interview or the decision to write an article, until one week after the publication of the interview or article. As of the date of this article, officers and/or employees of Streetwise Reports LLC (including members of their household) own securities of PolarityTE, a company mentioned in this article.
Disclosures from Cantor Fitzgerald, PolarityTE Inc., Initiation of Coverage, Feb. 14, 2018
Analyst Certification The analyst primarily responsible for this research report, and whose name appears on the front cover, certifies that: (i) all of the views expressed in this research report accurately reflects his or her personal views about any and all of the subject securities or issuers featured in this report; and (ii) no part of any of the research analyst’s compensation was, is, or will be, directly or indirectly related to the specific recommendations or views expressed by the research analyst in this report.
Legal Disclosures Investment banking (next 3 months):
Cantor Fitzgerald and/or its affiliates, expect to receive, or intend to seek, compensation for investment banking services within the next three months from all of the companies referenced within this report.
Cantor Fitzgerald and/or its affiliates is a market maker in PolarityTE Inc.
Cantor Fitzgerald and/or its affiliates is a market maker in Integra LifeSciences Holdings Corp.
CliveMaund.com Disclosure:
The above represents the opinion and analysis of Mr. Maund, based on data available to him, at the time of writing. Mr. Maund's opinions are his own, and are not a recommendation or an offer to buy or sell securities. Mr. Maund is an independent analyst who receives no compensation of any kind from any groups, individuals or corporations mentioned in his reports. As trading and investing in any financial markets may involve serious risk of loss, Mr. Maund recommends that you consult with a qualified investment advisor, one licensed by appropriate regulatory agencies in your legal jurisdiction and do your own due diligence and research when making any kind of a transaction with financial ramifications. Although a qualified and experienced stockmarket analyst, Clive Maund is not a Registered Securities Advisor. Therefore Mr. Maund's opinions on the market and stocks can only be construed as a solicitation to buy and sell securities when they are subject to the prior approval and endorsement of a Registered Securities Advisor operating in accordance with the appropriate regulations in your area of jurisdiction. Mr. Maund does not own shares of PolarityTE.


























































