The Life Sciences Report: Can you briefly describe your company? What makes Soligenix Inc. (SNGX:OTCQB) unique from other life sciences companies its size?
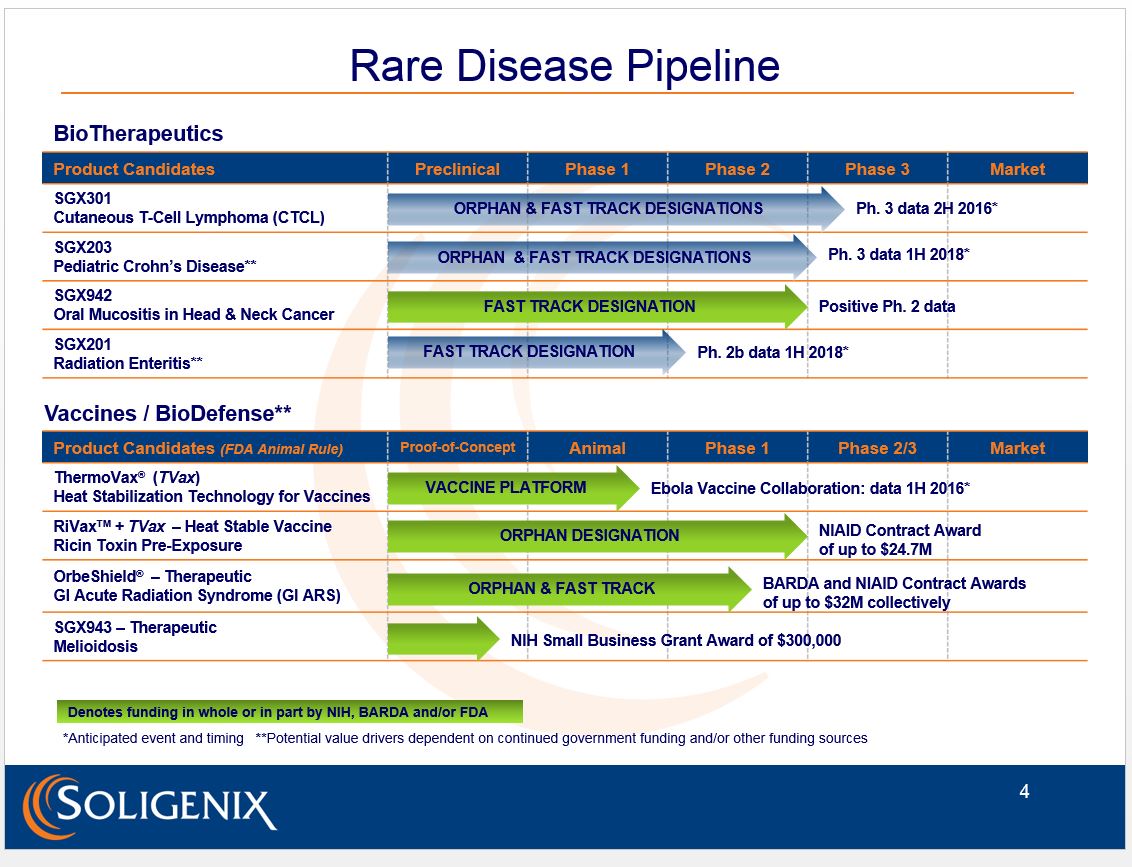
Christopher Schaber: Soligenix Inc. is a late-stage biopharmaceutical company focused on developing and commercializing products to treat rare diseases where there is an unmet medical need. You may ask whether this is truly unique, since a lot of companies are focused on rare diseases these days. That's true, but our rare or orphan disease focus spans both biotherapeutics and biodefense—we have been awarded up to $57 million ($57M) in government contract funding to develop our biodefense countermeasures. This has allowed us to develop a robust pipeline of eight programs—two in Phase 3, and another that has recently completed Phase 2—which is quite unique for a company our size. Moreover, the biodefense programs use some of the same active ingredients we pursue in our commercial programs, allowing for significant synergies.
TLSR: What programs on the biotherapeutic side are you and your team most excited about?
CS: There are actually three we are very excited about.
We have a cutaneous T-cell lymphoma (CTCL) program with SGX301 (synthetic hypericin), which is actively enrolling in a Phase 3, pivotal, double-blind, placebo-controlled study. In this study, topical synthetic hypericin is applied to cancerous skin lesions and activated with safe, visible fluorescent light. This study has been cleared through the FDA and results are currently expected in H2/16. Our synthetic hypericin has received orphan drug designation from both the FDA and the European health authorities. The FDA has also granted it fast-track status as a first-line treatment.
"Our rare or orphan disease focus spans both biotherapeutics and biodefense."
We have another Phase 3 pivotal study in pediatric Crohn's disease with SGX203 (beclomethasone dipropionate or BDP), which has also been cleared by the FDA. This double-blind study uses a novel oral formulation of immediate and delayed release BDP tablets to treat inflammation of the gastrointestinal tract. It is set to initiate in H2/16, with data expected in H1/18. Again, this program has FDA orphan drug designation, as well as fast-track status as a front-line treatment in mild to moderate pediatric Crohn's disease.
The third is our Phase 2 proof-of-concept, double-blind, placebo-controlled, dose-ranging study in oral mucositis in head-and-neck cancer with SGX942 (dusquetide). SGX942 is a novel, first-in-class, innate defense regulator that is administered during a patient's chemoradiation treatment as a short, four-minute, intravenous infusion. This study has recently reported positive preliminary results, which we are extremely excited about. We are now working on the pivotal Phase 3 trial design, and anticipate having a study that could support potential approval in both the U.S. and Europe before the end of the year. Moreover, given the biological activity of dusquetide, there are significant indication expansion opportunities in the long term. We are focused on oral mucositis in the short term.
TLSR: Oral mucositis is an area of unmet medical need, where there are no approved drugs. Let's focus on this proof-of-concept study. First, there have been other molecules tested for use in oral mucositis. What makes your molecule different?
CS: Dusquetide (the active ingredient in our SGX942 drug product for oral mucositis) is different for a number of reasons. It is a novel, first-in-class chemical entity, known as an innate defense regulator (IDR). It works at a key intracellular convergence point in the innate immune pathways, targeting a protein that no other compound has been shown to bind to.
Given the unique features of SGX942 as an IDR, it has the potential to address all stages of oral mucositis disease progression. Oral mucositis is generally described in five stages. For normal tissue, dusquetide would not have an impact because it only modulates activated innate immune pathways, and innate immunity is not yet activated. This attribute explains dusquetide's excellent safety profile.
Chemotherapy and radiation cause damage to cells. While chemoradiation is obviously focused on the tumor cells, some damage to the surrounding normal tissue is inevitable. SGX942 does not change this, and thus does not protect the tumor. This is very important in treating oral mucositis—the treatment needs to be focused on the surrounding tissue and not inadvertently protect the tumor.
"The biodefense programs use some of the same active ingredients we pursue in our commercial programs, allowing for significant synergies."
Immediately after chemoradiation causes damage, the damaged cells (tumor or not) release damage-associated molecular pattern molecules ("DAMPs") that activate the innate immune system, which initiates an inflammatory response. While necessary, the inflammatory signaling ultimately exacerbates the tissue damage. This is called the amplification phase. Because SGX942 changes the balance of the innate immune response, it may have a direct impact here, decreasing the inflammatory signaling—and therefore decreasing any additional damage—while also augmenting the "tissue healing" pathways.
Once some damage is done, the damaged area is susceptible to infection. Any infection will also exacerbate the damage. SGX942, again due to its ability to modulate the innate immune pathways, also augments the anti-infective pathways. The anti-infective and tissue healing pathways actually are intricately tied together, so one response is a consequence of the other.
Finally, when the tissue starts the healing process, dusquetide augments this process.
SGX942 is one of the only compounds described to date that can directly impact each of these stages without protecting the tumor as well. This is why we are so excited about the recent Phase 2 clinical trial results, as they clearly show the potential of SGX942.
TLSR: Treatment with SGX942 appeared to be most effective at the lower dose evaluated. Why do you think this might be? Isn't more usually better?
CS: Very good question. It is certainly true that when an enzyme is "misbehaving" and you want to turn it off, then the more drug you give, the more you "turn off" the enzyme and the better the drug works. This is true for most drugs, with the limitation being unwanted side effects. Unwanted side effects limit the use of even the most common drugs, such as aspirin.
However, immune modulators in general do not work this way. We are not attempting to "turn off" parts of the immune system; we are simply trying to rebalance the response, to redirect aspects of the response away from inflammation and toward other factors. A little inflammation is good: It is the danger signal to the rest of the body, and you don't want to turn it off completely. For example, when you use anti-TNF drugs and turn off TNF completely, patients become more susceptible to infection and lymphoma.
"We anticipate having a study that could support potential approval of SGX942 in both the U.S. and Europe before the end of the year."
Thus, with immune modulators, more is not always better. Rather, there is usually a dose level where the system is rebalanced correctly. Giving more of the compound potentially skews this balance, and the beneficial effect can disappear. It is important to note that based on our Phase 1 studies, the range of doses that rebalance appropriately is quite wide, and we have now identified 1.5 mg/kg as a very effective dose within this range for treating oral mucositis.
TLSR: In addition to the impact of dusquetide on oral mucositis, you also note a reduction in infection and an increase in tumor response. Were you surprised by these findings?
CS: As I noted previously, dusquetide has a very unique mechanism of action. It modulates the innate immune system. The innate immune system is very involved in both infection and in the tumor microenvironment—the cells surrounding the tumor that don't attack the tumor the way they should.
Our preclinical work was actually initiated in the infectious disease space, and we were expecting to see a signal in oral mucositis patients, although the incidence of infection in this patient population is somewhat limited, and our sample size was relatively small. We were very gratified to see a signal despite the small sample size.
With respect to tumor response, we have only investigated this in the most preliminary context with SGX942, and while we certainly don't protect the tumor, the true extent of our anti-tumor response will become more apparent with the 12-month follow-up data, expected late this year.
TLSR: I want to briefly touch on your Phase 3 cutaneous T-cell lymphoma trial, which is actively enrolling. I see that you are collaborating with the National Organization for Rare Disorders (NORD) and the Cutaneous Lymphoma Foundation (CLF) on the conduct of this study. Can you speak to how the organization is assisting you, and when you expect to have results?
CS: Sure. NORD and the CLF are assisting with the education and recruitment of patients for our pivotal Phase 3 clinical study of SGX301 (synthetic hypericin) in the treatment of CTCL. We hope that, with the assistance of these two very important patient groups, we can potentially accelerate enrollment into the study, and possibly complete it as soon as the end of 2016. Assuming positive results, we could potentially have FDA approval as soon as the end of 2017/the first half of 2018—not that far away, from a drug development perspective.
Of note: At some point, we also anticipate similar support from NORD and possibly the Crohn's and Colitis Foundation for our Phase 3 study in pediatric Crohn's disease with SGX203 (oral BDP).
TLSR: Are you looking for commercial partners, or do you plan to commercialize yourself?
CS: As you would imagine, we remain very open and opportunistic regarding partnership, and have a number of discussions taking place regarding potential collaborations at this time. However, we also realize that a number of our programs are highly specialized and, quite frankly, tailor-made for a small company like Soligenix to commercialize, especially in the U.S.
"We are in active business development with a number of companies regarding potential partnerships across our robust pipeline."
Obviously, biodefense is pretty straightforward, the one customer being the government. But even with CTCL or pediatric Crohn's disease, you are not talking about needing a very large commercial unit to move these forward—maybe 25 to 35 people. Therefore, at this time, we are evaluating the potential of maintaining the U.S. markets for these rare disease areas and commercializing ourselves as another important way to increase shareholder value.
TLSR: Is it possible to provide a bit more color on the status of your business development discussions, such as the companies you are speaking with, or how advanced discussions are?
CS: As you would imagine, I have been asked this question quite a bit. I really wish I could, but I must be very careful, given nothing is complete until you have a signed agreement. I can say that we are in very active discussions with a number of companies, and that we are hopeful we will be able to close on one or more in 2016. Rest assured, no one would like to talk about the business details more than me right now, but for legal reasons we cannot until it is official. It makes good sense for us, and for potential shareholders at the end of the day.
TLSR: Understood. Moving on: Can you address the market potentials for the Phase 3 programs in terms of estimated revenue and/or number of patients impacted for these drug candidates?
CS: For pediatric Crohn's, the number is about 160,000 patients worldwide annually, and we currently estimate this indication to be a ~$200M market opportunity. For CTCL, about 40,000 patients are diagnosed worldwide annually, and we estimate this indication to be a ~$250M market opportunity. We feel there is significant opportunity and value in our drug candidates, especially for a company like Soligenix.
TLSR: Can you briefly tell me what programs in your biodefense business segment you are most excited about?
CS: Sure. That would be our ThermoVax® vaccine heat stabilization platform technology, where we can take liquid vaccines of a certain construct and freeze-dry them so that they can be stored outside the refrigerator for extended periods of time, even at high temperature. Currently we have done this with our ricin vaccine, as well as with an anthrax vaccine and a human papillomavirus (HPV) vaccine.
In addition to ThermoVax®, we are excited about our ricin toxin vaccine, called RiVax™, which is supported by a National Institute of Allergy and Infectious Diseases (NIAID) contract of up to $24.7M. To date, we have shown we can provide 100% protection to nonhuman primates (NHPs) exposed to the aerosolized toxin. We have the potential to have the first approved ricin vaccine, as we are the world leaders in development here.
We are also excited about our oral BDP therapeutic, known as OrbeShield®, for the treatment of gastrointestinal acute radiation syndrome (GI ARS). With this particular program we have up to $32M of Biomedical Advanced Research and Development Authority (BARDA) and NIAID funding. Here, as well, we have demonstrated preliminary efficacy and safety in a large animal model.
In all, we have up to $57M of government funding across these key programs.
TLSR: Would you briefly summarize the major takeaways for investors?
CS: Yes. We have two Phase 3 pivotal programs that will support potential FDA approvals in orphan diseases, both fast-tracked by FDA. Results expected in CTCL as early as H2/16, and results in pediatric Crohn's disease expected in H1/18.
We also recently announced positive preliminary results from a Phase 2 proof-of-concept study in oral mucositis in head-and-neck cancer with SGX942, which has a unique, first-in-class mechanism of action.
We are in active business development with a number of companies regarding potential partnerships across our robust pipeline. And we are also evaluating the potential to commercialize the rare diseases product candidates ourselves in the U.S.
We have a vaccine/biodefense business funded up to $57M by the government that almost entirely supports the development of RiVax™, a heat-stable ricin toxin vaccine, and OrbeShield®, a therapeutic for gastrointestinal acute radiation syndrome. Both have positive data to date, demonstrating potential efficacy and safety. ThermoVax®, our propriety vaccine heat stabilization platform technology, has demonstrated that we can stabilize our ricin vaccine, as well as HPV and anthrax vaccines, such that they no longer require refrigerated storage.
TLSR: Is there anything additional you would like a potential investor in Soligenix to know?
CS: No. I think we covered the main points. I'd just like to conclude by asking your readers to please take a moment to check out the Soligenix corporate website, where they will find interesting and useful information.
TLSR: Thank you for the time today.
Christopher J. Schaber, Ph.D., has more than 25 years of experience in the pharmaceutical and biotechnology industry. Dr. Schaber has been the Soligenix president, chief executive officer and a director since August 2006. He was appointed chairman of the board in 2009. He also serves on the boards of directors of the Biotechnology Council of New Jersey and the Alliance for Biosecurity, and has been a member of the corporate councils of both the National Organization for Rare Diseases and the American Society for Blood and Marrow Transplantation. Prior to joining Soligenix, Dr. Schaber served from 1998 to 2006 as executive vice president and COO of Discovery Laboratories Inc., where he was responsible for overall pipeline development and key areas of commercial operations, including regulatory affairs, quality control and assurance, manufacturing and distribution, preclinical and clinical research, and medical affairs, as well as coordination of commercial launch preparation activities. From 1996 to 1998, Dr. Schaber was a cofounder of Acute Therapeutics Inc., and served as its vice president of regulatory compliance and drug development. From 1994 to 1996, Dr. Schaber was employed by Ohmeda PPD Inc. as worldwide director of regulatory affairs and operations. From 1989 to 1994, Dr. Schaber held a variety of regulatory, development and operations positions with The Liposome Company Inc., and Elkins-Sinn Inc., a division of Wyeth-Ayerst Laboratories. Dr. Schaber received his bachelor's degree from Western Maryland College, his master's degree in pharmaceutics from Temple University School of Pharmacy and his Ph.D. in pharmaceutical sciences from the Union Graduate School.
Read what other experts are saying about:
Want to read more Life Sciences Report interviews like this? Sign up for our free e-newsletter, and you'll learn when new articles have been published. To see a list of recent interviews with industry analysts and commentators, visit our Interviews page.
Disclosure:
1) Tracy Salcedo compiled this interview for Streetwise Reports LLC and provides services to Streetwise Reports as an employee. She owns, or her family owns, shares of the following companies mentioned in this interview: None.
2) Soligenix Inc. paid Streetwise Reports to produce and distribute this interview. Streetwise Reports does not accept stock in exchange for its services. Click here for important disclaimers. The information provided above is for informational purposes only and is not a recommendation to buy or sell any security.
3) Christopher Schaber had final approval of the content and is wholly responsible for the validity of the statements. Opinions expressed are the opinions of Christopher Schaber and not of Streetwise Reports or its officers. I was not paid by Streetwise Reports to participate in this interview. I had the opportunity to review the interview for accuracy as of the date of the interview and am responsible for the content of the interview. I or my family own shares of the following companies mentioned in this interview: Soligenix Inc. My company has a financial relationship with the following companies mentioned in this interview: None.
4) Interviews are edited for clarity. Streetwise Reports does not make editorial comments or change experts' statements without their consent.
5) The interview does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports' terms of use and full legal disclaimer.
6) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned. Directors, officers, employees or members of their families are prohibited from making purchases and/or sales of those securities in the open market or otherwise during the up-to-four-week interval from the time of the interview until after it publishes.