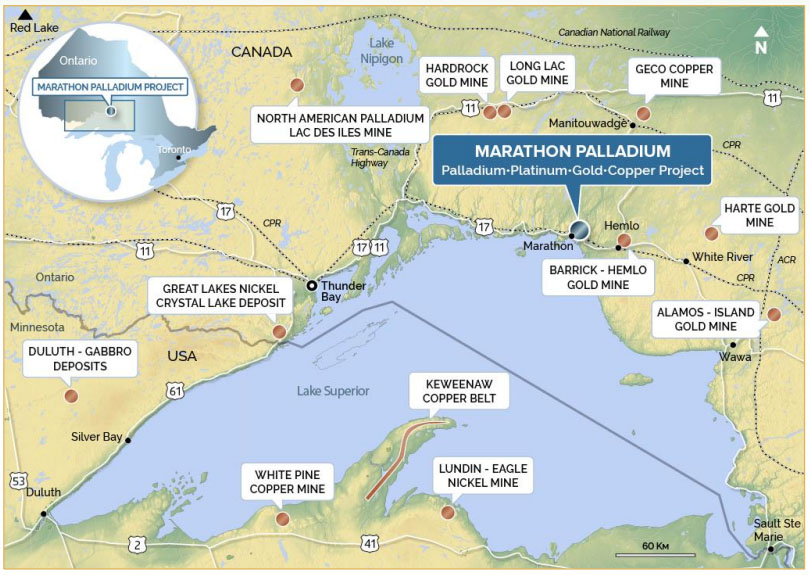
While many in Northwestern Ontario look to the Ring of Fire and chromite as a solid way of boosting our regionís economy, there are more minerals in our region that are generating a great deal of excitement. One of those minerals is graphite.
Why?
Graphite can be made into graphene. The origin of the word, Graphene, was coined as a combination of graphite and the suffix -ene, which means molecules with a double bond of carbon. Its structure is one-atom-thick (0.35nm) planar sheets of sp2-bonded carbon atoms that are densely packed in a honeycomb crystal lattice. It is Fullerene if carbon atom is in the form of sphere, Carbon Nanotube if it is in cylindrical nanostructure, and Graphene if it is unfolded.
Graphene is twice stronger than diamond, and 200 times stronger than steel. Also, diamond is a nonconductor that does not conduct electricity, but graphene conducts electricity very well, and has electrical conductivity that is more than 100 times higher than copper. For example, if light travels at the constant speed of 300,000km in a vacuum, electron travels at the constant speed of 1000km/sec in graphene.
The reason for the difference is the difference in the bonding that connects carbon and carbon. Compare to diamond, a single bond, graphene has a double bond. As the name implies, double bond has two bridges connecting carbon and carbon. Because double bond is a stronger bond than a single bond, which has one bridge to connect carbon and carbon, graphene is stronger than diamond when compared and through one of two bonds, electrons can go back and forth between graphene.
It also means diamond, which has a single bond, does not conduct electricity, but graphene does. . .View Full Article