Abiomed Shares Gain 8% After Firm Announces Record Q3 Revenue of $232 Million
Source: Streetwise Reports (01/28/2021)
 Shares of cardiac medical device maker Abiomed Inc. traded higher after the company reported record revenue for Q3/21 despite the impact from COVID-19.
Shares of cardiac medical device maker Abiomed Inc. traded higher after the company reported record revenue for Q3/21 despite the impact from COVID-19.
read more >
Avinger Shares Rise 40% on Launch of Image-Guided Device to Diagnose and Treat PAD
Source: Streetwise Reports (01/15/2021)
 Shares of Avinger Inc. reached a new 52-week high after the company reported it initiated a full commercial launch of its Tigereye™ Image-Guided CTO Crossing Catheter system for diagnosing and treating peripheral artery disease.
Shares of Avinger Inc. reached a new 52-week high after the company reported it initiated a full commercial launch of its Tigereye™ Image-Guided CTO Crossing Catheter system for diagnosing and treating peripheral artery disease.
read more >
Inari Shares Flow 14% Higher on Triple Digit YoY Growth in Procedures and Revenues
Source: Streetwise Reports (01/13/2021)
 Shares of Inari Medical Inc. established a new 52-week high after the company reported that preliminary revenue in Q4/20 increased year-over-year by greater than 140%.
Shares of Inari Medical Inc. established a new 52-week high after the company reported that preliminary revenue in Q4/20 increased year-over-year by greater than 140%.
read more >
Biotech Collaborates to Advance Psychedelics Use in Cancer, Other Applications
Source: Streetwise Reports (11/23/2020)
 Revive Therapeutics enters an agreement with PharmaTher, a specialty psychedelics pharmaceutical firm.
Revive Therapeutics enters an agreement with PharmaTher, a specialty psychedelics pharmaceutical firm.
read more >
Glaukos Shares Pressured Higher After Firm Reports 11% Rise in Q3 Revenue
Source: Streetwise Reports (11/06/2020)
 Shares of Glaukos Corp. traded 9% higher after the company reported Q3/20 financial results that included an 11% YoY increase in glaucoma and corneal health net sales.
Shares of Glaukos Corp. traded 9% higher after the company reported Q3/20 financial results that included an 11% YoY increase in glaucoma and corneal health net sales.
read more >
Inspire Medical's Shareholders Rest Comfortably on Q3 Earnings and Raised FY Outlook
Source: Streetwise Reports (11/03/2020)
 Shares of Inspire Medical Systems traded 30% higher and set a new 52-week high after the company reported Q3/20 financial results that included a 72% rise in YoY revenue.
Shares of Inspire Medical Systems traded 30% higher and set a new 52-week high after the company reported Q3/20 financial results that included a 72% rise in YoY revenue.
read more >
Align Technology Shares Head Straight Up on 20.9% Q3 Revenue Growth as Firm Celebrates its 9 Millionth Invisalign Patient
Source: Streetwise Reports (10/22/2020)
 Shares of Align Technology traded 35% higher and climbed to a new 52-week high after the company reported Q3/20 financial results that included a 20.9% YoY increase in net revenues.
Shares of Align Technology traded 35% higher and climbed to a new 52-week high after the company reported Q3/20 financial results that included a 20.9% YoY increase in net revenues.
read more >
U.S. Medical Device Firm to Acquire Developer of Dissection Stent
Source: Streetwise Reports (09/09/2020)
 The specifics of the deal and the next steps for CryoLife are presented in a Ladenburg Thalmann report.
The specifics of the deal and the next steps for CryoLife are presented in a Ladenburg Thalmann report.
read more >
AI Tech Firm Provides COVID-19 Contact Tracing Solution Update
Source: Streetwise Reports (07/20/2020)
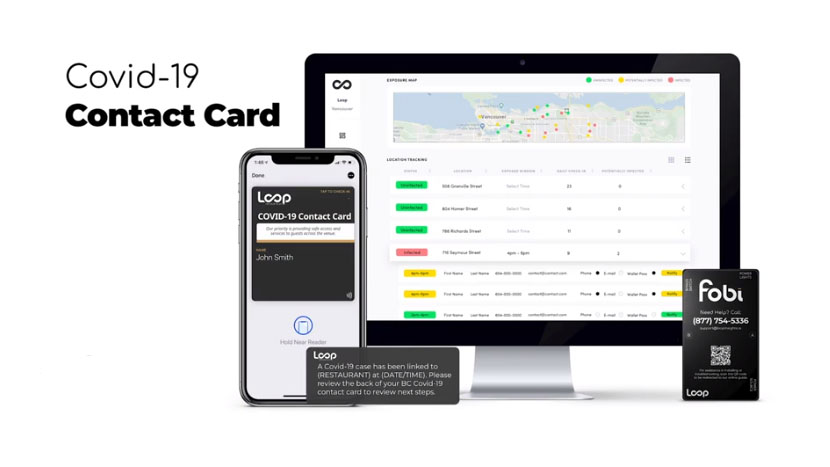 Loop Insights continues to educate governments and venue operators around the world about its easily implemented platform.
Loop Insights continues to educate governments and venue operators around the world about its easily implemented platform.
read more >
Announcing Streetwise Live! Events
Source: Streetwise Reports (07/13/2020)
 Streetwise Reports is offering 100 investors—at no charge—the opportunity to meet via Zoom with executives of precious metals companies as they discuss recent developments at their companies and upcoming catalysts.
Streetwise Reports is offering 100 investors—at no charge—the opportunity to meet via Zoom with executives of precious metals companies as they discuss recent developments at their companies and upcoming catalysts.
read more >




























































