Last week, the US Federal Trade Commission (FTC) suspended its legal challenge to Amgen’s proposed acquisition of Horizon Therapeutics, 2022’s largest deal announcement in the biopharma space. The FTC and Amgen-Horizon then agreed to settle on Friday, clearing the way for the $27.8 billion purchase to close sometime in the fourth quarter. The FTC notes that, as part of the settlement, attorneys general from six states – California, Illinois, Minnesota, New York, Washington, and Wisconsin – will also dismiss a related federal court preliminary injunction action.
There was significant doubt cast upon the FTC’s case not long after the commission originally filed its suit in May, given its employment of a novel theory that Amgen could eventually bundle its drugs with those it is acquiring from Horizon in negotiations with insurers – therefore entrenching Horizon products’ premiere placement in the market and choking out potential competitors that might be cheaper or more effective. Horizon currently sells two marketed products, Tepezza (teprotumumab) for thyroid eye disease and Krystexxa (pegloticase), a chronic refractory gout treatment. Since Amgen was quick to agree that they would not bundle their products with Horizon’s, a settlement was the natural conclusion. Further conditions of the agreement between the FTC and the two firms stipulate Amgen will not introduce discount or rebate schemes on their own products that would influence the sale or positioning of Horizon’s drugs.
The FTC, which has become more aggressive toward mega-mergers across multiple industries, presented the Amgen-Horizon settlement as a win, but it seems more likely that an ongoing wave of M&A activity among pharmaceutical and biotechnology firms will be bolstered by the sudden conclusion of the suit.
GlobalData’s Deals Database, cited by Pharmaceutical Technology, notes that there were 479 pharma M&A deals announced in Q2 2023, increasing by 18% QoQ in Q2, and 151% YoY. The total value of these deals was $51 billion, decreasing by -30% in Q2, compared with the previous quarter’s total of $72.5 billion. Still, Q2’s pharma industry M&A deal value rose by 77% YoY.
Though fewer deals were signed in the first quarter than in the second, the size of Q1’s deal value was boosted by Pfizer Inc.’s announcement that they would be acquiring massive biotech firm Seagen Inc. in biopharma’s largest deal in almost four years’ time. Pfizer’s offer of $229 per share in cash, a 33% premium on Seagen’s share price at the close preceding the deal becoming public, pushed total value of the deal to $43 billion.
As MRP has previously noted, Pfizer executives have been among a consortium of biopharma heads that have voiced their desire to increase dealmaking with outside companies. Pfizer has set a goal of adding $25 billion in revenue by 2030 from business-development moves including acquisitions. Those could help the company offset an estimated drop of roughly $17 billion in sales from upcoming patent expirations. Bloomberg notes that Pfizer thinks that sales of Seagen’s four FDA-approved oncology products will exceed $10 billion, about $2 billion more than analysts’ estimates.
The smooth closure of the Pfizer-Seagen tie-up is still beholden to regulators at the FTC, but the recent news on the Amgen-Horizon deal is likely to invigorate confidence among investors that this deal will ultimately receive approval as well. As of July, the FTC requested more information from Pfizer and Seagan in their review of the deal. Fierce Pharma writes that second requests from the FTC occur in roughly 25% of M&A deals, citing MEDACorp data. The regulator challenges such a transaction 5% – 10% of the time.
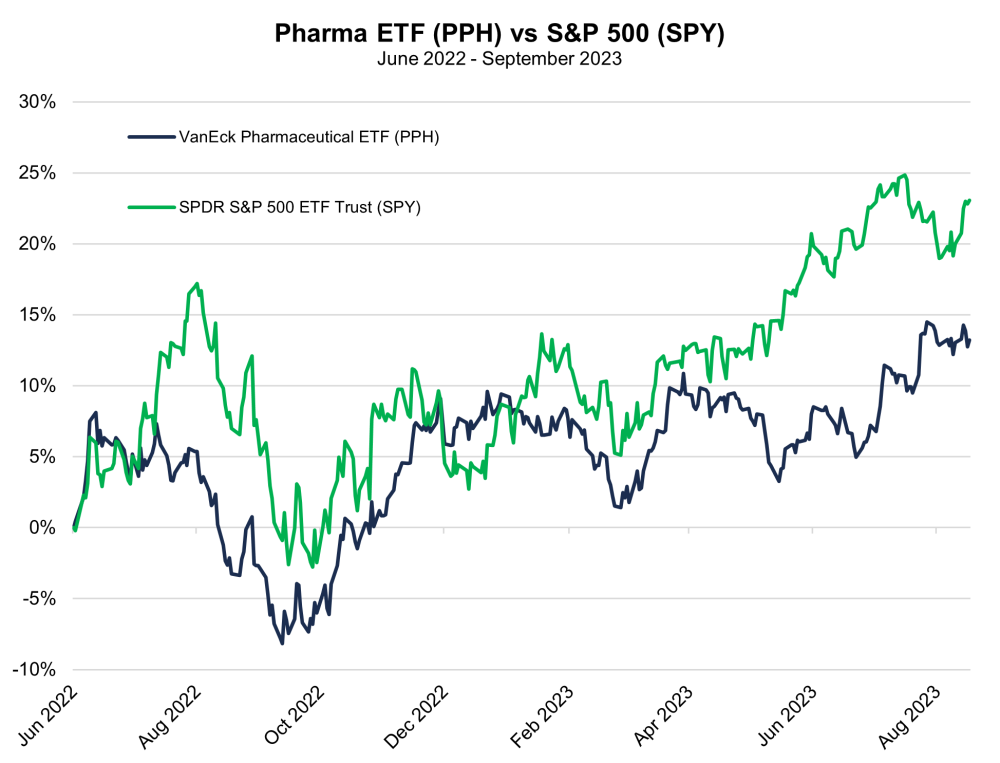
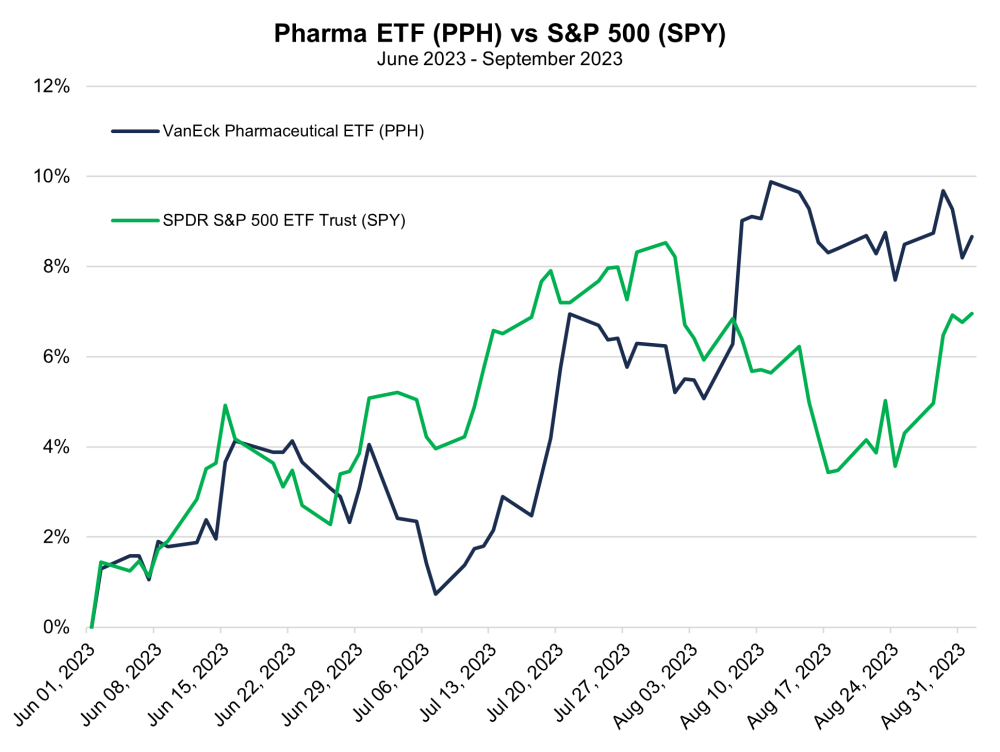
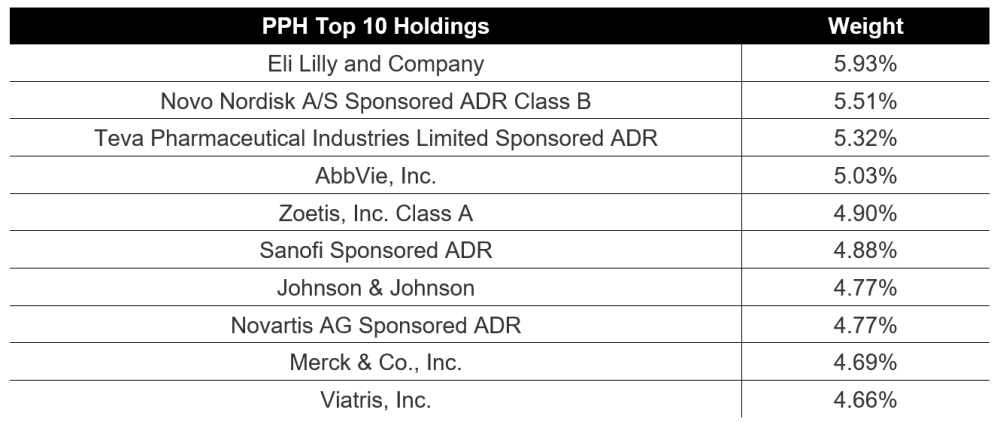
Charts