Many of us have resigned ourselves to thinking the pandemic will never end and that after a couple more flurries of variants the disease might mellow out and turn into just an endemic problem. The Biden administration is calling this strategy living with the virus, and many nation states are following this lead; but It's hard to believe this is acceptable given all our technology and resources. Do we really lack the ability to make a pill that stops death and the spread of this pandemic?
Big pharma players like Pfizer Inc. (PFE:NYSE), BioNTech SE (BNTX:NASDAQ), Johnson & Johnson (JNJ:NYSE), Merck & Co. Inc. (MRK:NYSE), and Moderna Inc. (MRNA:NASDAQ) were all involved in developing a vaccine that was quickly obsoleted by a number of new variants that have emerged that are clearly evading the protection of the vaccine.
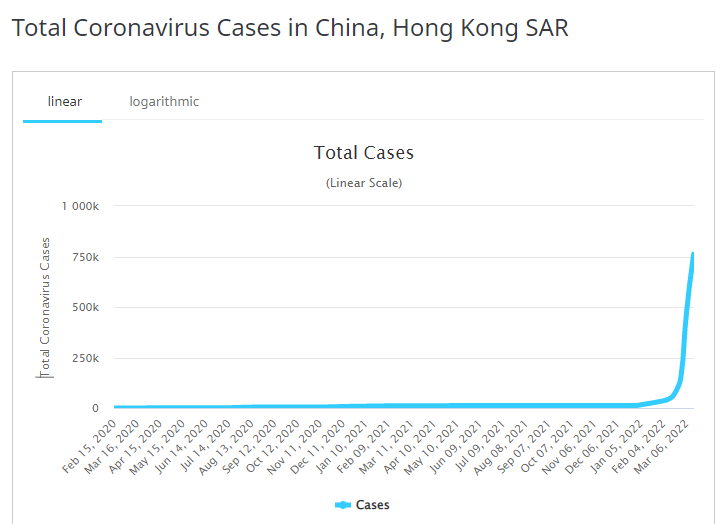
Look at the swells in new cases in Zero Covid states like Hong Kong that went from 12,600 total cases since the pandemic inception to 750,000+ in 2 months. Countries like Hong Kong, frustrated with controlling the spread of the virus, are having to modify their strategy and no longer send mild cases to isolation centers.
The once high-flying vaccine megacaps have gotten eviscerated in the stock market. Surely one of these big pharmas would have been smart enough to see this pivot coming and invest in a therapeutic with robust efficacy and reasonable convenience.
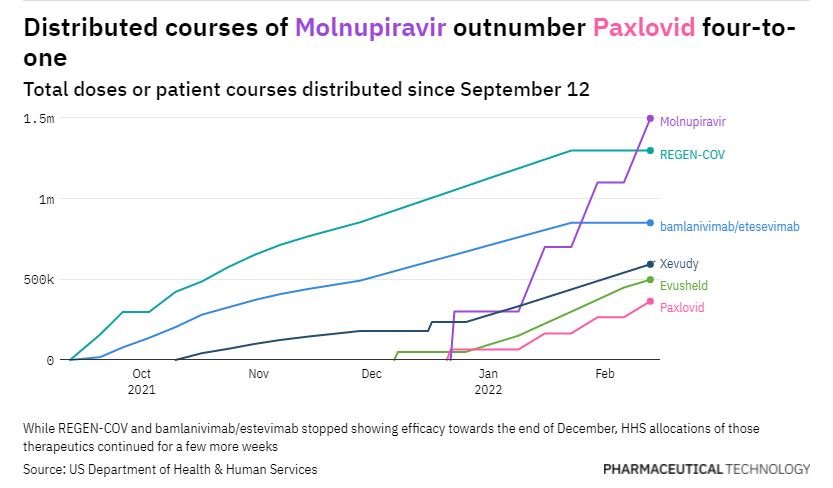
Out of all these names, Pfizer was arguably the only one to truly innovate with its potent 3CL protease inhibitor Paxlovid which works great if you give it to people within 5 days of symptoms.
However, the reality of the situation is that the requirements of an underlying condition, a prescription, and a positive COVID-19 test limit the widespread use of the drug. In the interim, molnupiravir, Merck’s drug with very low efficacy and mutagenic properties is being substituted.
Pfizer Was Not the First Therapeutic to Take Dying off the Table
A small biotech by the name of CytoDyn Inc. (CYDY:OTCQB) had a monoclonal antibody called leronlimab that showed efficacy very early in the pandemic. In March 2020 before remdesivir approval they made a news splash when their anecdotal stories hit the NY Post.
In final analysis, their monoclonal antibody reduced 14 day mortality by 82% but their slow recruitment, poor clinical trial design, and an ineffective Drug Safety Monitoring Board caused a massive delay in the potential approval of this drug.
Leronlimab could have saved hundreds of thousands of lives had it received an EUA in March 2021 instead of remdesivir. This was a perfect storm in a series of unfortunate events that stymied FDA approval.
Now the company’s trajectory is a bit unclear with their current funding, CRO, and clinical development issues that will need to be navigated. However, the drug’s potential remains very promising, encompassing fields from metastatic cancer to HIV to other inflammatory indications.
Moving on to another COVID-19 therapeutic that demonstrated efficacy in the hospitalized setting is GB0139 which is a drug delivered by inhaler that is manufactured by Galecto Inc. (NASDAQ: GLTO). GB0139 is a galectin-3 inhibitor which intends to break the fibrotic feedback loop experienced by hospitalized COVID-19 patients.
This phase 2b study showed that it was safe to take the drug and that inflammatory biomarkers rapidly decreased while also reducing organ damage and mortality by 21%. However, a 21% improvement is modest given the statistical power of a subgroup from a 40 patient study.
Another promising pill being developed for mild-to-moderate cases of COVID-19 is Prolectin-M, a galectin antagonist made by BioxyTran (OTCMKTS: BIXT). This company is currently a caveat emptor so the shares cannot be traded right now, but this doesn’t detract from the science and their clinical progress, as well as the fact that they are a full SEC reporting company and pink current.
This pill is a complex carbohydrate and typically drugs like this have fantastic safety profiles, can be manufactured at scale, and are great candidates for widespread use. BioxyTran’s preclinical studies show robust elimination of SARS-CoV-2, and in a small phase 1/2 study Prolectin-M reduced viral loads to undetectable levels within 3 days, which is considered negative for disease. Prolectin-M is expected to enroll patients in a phase 3 clinical trial.
The Therapeutic That Bested Remdesivir
It actually synergized with remdesivir but also, according to studies to date, had a much greater clinical effect as measured by 7 day reduction in hospitalization days and mortality. This drug, studied in the hospitalized setting is called Tollovir. It is produced by Todos Medical (TOMDF:OTCQB), and in a phase 2 trial it reduced COVID-19 related deaths by 100% as well as reduced hospital stays by about 7 days. This product also has a “cousin” nutraceutical called Tollovid which is basically a cousin OTC product of the drug Tollovir, a less potent version of the natural product.
One thing that makes Todos’ strategy so novel is that they are now “preparing to gather data based on real world evidence to upgrade Tollovid’s regulatory status from dietary supplement to OTC drug targeting adult outpatient treatment, pediatric outpatient treatment and adult & pediatric Long COVID,” according to their latest press release.
These products come from the same natural source and so one’s data might even support the other.
Despite Promise, Other Antivirals Run Into Development Issues
For instance, MultiStem, an allogeneic adult stem cell therapy developed by Athersys Inc. (ATHX:NASDAQ), is not yet ready for mass manufacturing and rollout despite very promising clinical data in the ONE-BRIDGE and MUST-ARDS studies of all-cause ARDS, COVID-induced ARDS (100% mortality benefit, n=5), and ischemic stroke. Development remains ongoing and very promising but the company’s focus is on all-cause ARDS and ischemic stroke, not specifically COVID-19.
Another promising competitor was INmune Bio Inc. (INMB:NASDAQ). INmune was developing its soluble TNF inhibitor, Xpro1595, for hospitalized cases of COVID-19 but the program was deprioritized.
Lastly, other companies such as Adamis Pharmaceuticals Corp. (ADMP:OTCBB) and Atea Pharmaceuticals Inc. (AVIR:NASDAQ) have lagged in development with relatively slow development, having a lack of clinical data and not proving clinical superiority to remdesivir, respectively.
There is a little bit of hope for Humanigen Inc. (HGEN:NASDAQ) immune modulator lenzilumab, as it had a rejected emergency use authorization (EUA) attempt, but the company remains committed to submitting more data that may support an EUA or full approval.
There are really a handful of companies with novel approaches and truly focusing on COVID-19 and being able to provide timely solutions — but the companies and solutions providing promising oral antivirals for outpatient use are Todos (given Pfizer’s data) and BioxyTran.
Without trying to be an alarmist, the next COVID-19 surge truly has the potential to be more deadly and infectious than the last. The large number of infections across the globe but particularly in areas such as Hong Kong has almost ensured that the next variant has already arrived and is just lurking in the shadows.
Unfortunately approval of two promising oral antiviral candidates, Prolectin and Tollovir, will not make it in time for the next wave. But investors and consumers nonetheless need to stay focused on antiviral preparedness to mitigate the potential damage of any new strain of COVID-19.
Disclosures
1) Vision and Value: I, or members of my immediate household or family, own securities of the following companies mentioned in this article: Athersys Inc., CytoDyn Inc., INmune Bio Inc., and Todos Medical. I personally am, or members of my immediate household or family are, paid by the following companies mentioned in this article: None. I determined which companies would be included in this article based on my research and understanding of the sector.
2) The following companies mentioned in this article are billboard sponsors of Streetwise Reports: Todos Medical. Click here for important disclosures about sponsor fees.
3) Statements and opinions expressed are the opinions of the author and not of Streetwise Reports or its officers. The author is wholly responsible for the validity of the statements. The author was not paid by Streetwise Reports for this article. Streetwise Reports was not paid by the author to publish or syndicate this article. Streetwise Reports requires contributing authors to disclose any shareholdings in, or economic relationships with, companies that they write about. Streetwise Reports relies upon the authors to accurately provide this information and Streetwise Reports has no means of verifying its accuracy.
4) This article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports' terms of use and full legal disclaimer. This article is not a solicitation for investment. Streetwise Reports does not render general or specific investment advice and the information on Streetwise Reports should not be considered a recommendation to buy or sell any security. Streetwise Reports does not endorse or recommend the business, products, services or securities of any company mentioned on Streetwise Reports.
5) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned. Directors, officers, employees or members of their immediate families are prohibited from making purchases and/or sales of those securities in the open market or otherwise from the time of the decision to publish an article until three business days after the publication of the article. The foregoing prohibition does not apply to articles that in substance only restate previously published company releases.
6) This article does not constitute medical advice. Officers, employees and contributors to Streetwise Reports are not licensed medical professionals. Readers should always contact their healthcare professionals for medical advice.