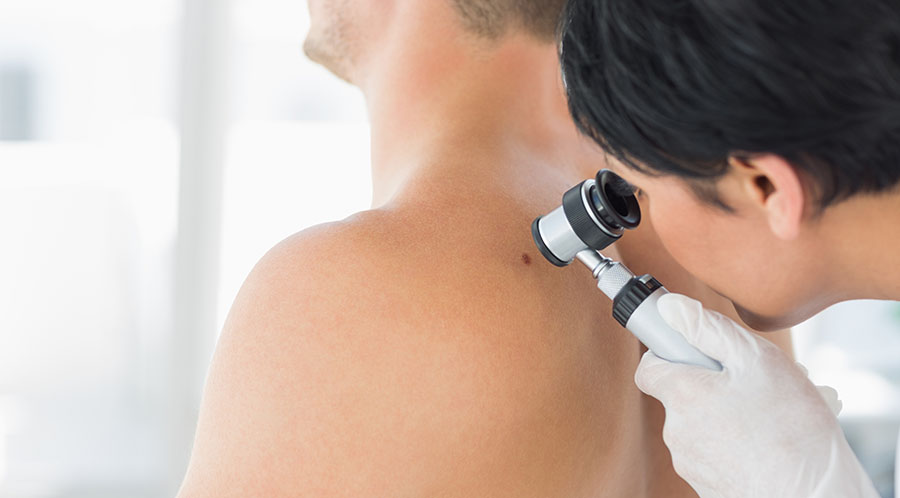
Molecular dermatology company DermTech Inc. (DMTK:NASDAQ), which is focused on delivering precision dermatology utilizing a non-invasive skin genomics platform, today announced that "Geisinger Health System has issued a positive medical benefit policy for its Commercial and Medicare Business Segment for the DermTech Pigmented Lesion Assay (PLA)."
The company stated that its PLA is "the first non-invasive gene expression test for the early detection of melanoma." DermTech further explained that "PLA has a 99% negative predictive value (NPV), meaning there is a less than 1% probability of the PLA missing a melanoma when administered properly."
The firm indicated that Geisinger Health System's policy aligns closely with the Medicare Administrative Contractor, Palmetto GBA MolDx. The company noted that under these established guidelines, gene expression profiling for cutaneous melanoma utilizing the Pigmented Lesion Assay RNA gene expression test on skin samples obtained via adhesive patches is permissible and deemed to be medically necessary if certain criteria are met. The firm noted the specific criteria pertains to such things as the lesion's size, color, shape, diameter, location and evolving change. Additional parameters include if the lesion is ulcerated or bleeding, is observed in combination with other skin conditions, such as psoriasis, eczema or similar skin conditions, or if melanoma is highly suspected.
DermTech's Senior Vice President of Payor Access Dan Visage commented, "We are thrilled that Geisinger Health System, an organization with a well-known commitment to quality healthcare delivery and innovation, reviewed the clinical dossier and peer-reviewed publication library for the PLA and issued a positive medical benefit policy. Using the PLA will enhance the early detection of melanoma sparing the patient the need for an invasive biopsy."
In a separate recent news release, the company announced that its management team will be making a presentation discussing the details and benefits of its skin genomics testing platform to the 23rd annual ICR Conference 2021 on Thursday, January 14, 2021, at 10:00 am EDT. The firm noted that the full presentation may be viewed at the following link.
DermTech is a genomics company headquartered in La Jolla, Calif., specialized in dermatology. The firm states that it is inventing a new category of medicine for precision dermatology that offers a non-invasive skin genomics testing platform to detect skin cancer. The company is focused on developing more accurate diagnosis and treatment of melanoma and thereby reducing and eliminating unnecessary surgical biopsies and procedures. The result of enhanced detection methods is improvement in both patient care and lower costs. Rather than using a scalpel to cut away a skin sample for analysis, skin samples are collected and analyzed non-invasively using a simple adhesive patch resembling a band-aid. In addition to skin cancer detection, DermTech is also developing products for the assessment of inflammatory diseases and customized drug treatments.
DermTech began the day with a market capitalization of around $548.9 million with approximately 19.6 million shares outstanding and a short interest of about 4.9%. DMTK shares opened nearly 7% higher today at $29.90 (+$1.89, +6.75%) over yesterday's $28.01 closing price and reached a new 52-week high this morning of $36.00. The stock has traded today between $29.85 and $37.15 per share and is currently trading at $37.12 (+$9.11, +32.52%).
[NLINSERT]Disclosure:
1) Stephen Hytha compiled this article for Streetwise Reports LLC and provides services to Streetwise Reports as an independent contractor. He or members of his household own securities of the following companies mentioned in the article: None. He or members of his household are paid by the following companies mentioned in this article: None.
2) The following companies mentioned in this article are billboard sponsors of Streetwise Reports: None. Click here for important disclosures about sponsor fees.
3) Comments and opinions expressed are those of the specific experts and not of Streetwise Reports or its officers. The information provided above is for informational purposes only and is not a recommendation to buy or sell any security.
4) The article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports' terms of use and full legal disclaimer. This article is not a solicitation for investment. Streetwise Reports does not render general or specific investment advice and the information on Streetwise Reports should not be considered a recommendation to buy or sell any security. Streetwise Reports does not endorse or recommend the business, products, services or securities of any company mentioned on Streetwise Reports.
5) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned. Directors, officers, employees or members of their immediate families are prohibited from making purchases and/or sales of those securities in the open market or otherwise from the time of the decision to publish an article until three business days after the publication of the article. The foregoing prohibition does not apply to articles that in substance only restate previously published company releases.
6) This article does not constitute medical advice. Officers, employees and contributors to Streetwise Reports are not licensed medical professionals. Readers should always contact their healthcare professionals for medical advice.