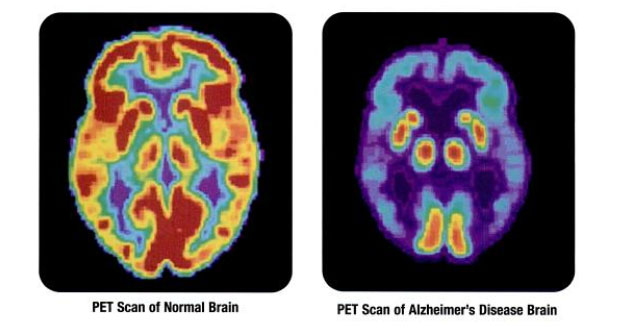
3D Signatures Inc. (DXD:TSX.V; TDSGF:OTCQB; 3D0:FSE) reported the results of its second clinical study of the TeloView platform in the degenerative brain disease in a March 21 press release. The results of the study have been accepted for publication in the Journal of Alzheimer's Disease.
Because the TeloView Alzheimer's test is both accurate and minimally invasive, it represents a disruptive diagnostic and prognostic tool for a disease that affects five million Americans over the age of 65, according to the company.
In the press release, Dr. Sabine Mai, 3D Signatures' cofounder and principal inventor, stated that, at present, Alzheimer's disease (AD) "is only confirmed postmortem pathologically. There is a significant need for an accurate, non-invasive biomarker that can diagnose AD and indicate disease progression, and we believe TeloView has the potential to answer that important call."
"As we understand it there's more than 1,000 drugs in development and working toward FDA approval right now for Alzheimer's and there's no test to tell if they work," said Jason Flowerday, 3D Signatures' CEO. "There's a lot of resources being devoted to finding therapies that are effective, and we're trying to develop a tool that will enable companies to identify better drugs, select appropriate patients for trials, monitor patients during trials and ultimately get those drugs to market."
If the 3D Signatures' platform proves its utility in additional clinical studies, company representatives told The Life Sciences Report the technology could be easily adopted by large pharma and biotech companies looking to ensure patients enrolled in clinical trials in Alzheimer's drug development truly have the disease, and could also be an accurate and cost-effective way to monitor progression of the disease in those drug trials.
The company is "exploring opportunities to expand the scope of its AD related work with further clinical studies and to fund that work through non-dilutive or independent financing arrangements, such as a joint venture," the press release states.
Commenting on the news, Knight Therapeutics CEO and president Jonathan Goodman stated, "Knight is delighted with its investment in 3D Signatures on multiple dimensions. One example is 3D Signatures' progress in Alzheimer's disease, including the company's ability to detect the presence and severity of the disease in living patients from a simple cheek swab. If validated through additional research, this could be a game-changer for AD diagnosis and the development of effective treatments."
Knight has provided "capital to advance the development of [3D Signatures'] novel diagnostic and prognostic technologies," according to a press release from December 2016.
"The search for sensitive and noninvasive markers to detect Alzheimer's has been a hot topic lately," Magdalena Kegel wrote in an article for Alzheimer's News Today about the 3D Signatures announcement. "For now, scientists can only make a definitive diagnosis of Alzheimer's after death, and amyloid plaques are detected in a patient's brain. And although current imaging methods can detect the Alzheimer's protein in the brains of living people, doctors don't consider this method practical for routine screening."
Given the large unmet medical need, the growing demographics of people susceptible to developing Alzheimer's, and consequently the large market opportunity, a number of large pharmaceutical firms are looking for an Alzheimer's treatment and/or cure. According to an article in PharmaExec.com, research firm GlobalData anticipates "The global market for Alzheimer's Disease (AD) treatment will more than double in value from $4.9 billion in 2013 to reach an estimated $13.3 billion by 2023."
In an article on the disease for Men's Health, Dr. Sanjay Asthana, director of the Wisconsin Alzheimer's Disease Research Center, notes that scientists believe the disease "will affect up to 13.8 million people by the year 2050, at an enormous cost—about $236 billion per year in health care, long-term care, and hospice expenses."
But progress on developing compounds that slow progression of the disease, or potentially cure it, is punctuated by failures. In a February 15 article on the failure of a candidate from Merck due to lack of efficacy, CNN.com also noted that Eli Lilly had halted a drug trial in the disease. But companies including AstraZeneca, Novartis, Amgen and Biogen continue to pursue treatments and potential cures.
In other news, 3D Signatures announced on March 29 that it has "successfully completed internal analytical assay validation for its Hodgkin's lymphoma ('HL') test ('Telo-HL') pursuant to FDA guidelines." The test is designed to stratify patients as relapsing or non-relapsing, enabling physicians to administer "more targeted and effective therapies" upon diagnosis.
Read what other experts are saying about:
Want to read more Life Sciences Report articles like this? Sign up for our free e-newsletter, and you'll learn when new articles have been published. To see recent articles and interviews with industry analysts and commentators, visit our Streetwise Interviews page.
Disclosure:
1) Tracy Salcedo compiled this article for Streetwise Reports LLC and provides services to Streetwise Reports as an independent contractor. She owns, or her family owns, shares of the following companies mentioned in this interview: None. She is, or members of her immediate household or family are, paid by the following companies mentioned in this article: None.
2) 3D Signatures Inc. is a billboard sponsor of Streetwise Reports. Streetwise Reports does not accept stock in exchange for its services. Click here for important disclosures about sponsor's fees. The information provided above is for informational purposes only and is not a recommendation to buy or sell any security.
3) Comments and opinions expressed are those of the specific experts and not of Streetwise Reports or its officers.
4) The article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports' terms of use and full legal disclaimer. This article is not a solicitation for investment. Streetwise Reports does not render general or specific investment advice and the information on Streetwise Reports should not be considered a recommendation to buy or sell any security. Streetwise Reports does not endorse or recommend the business, products, services or securities of any company mentioned on Streetwise Reports.
5) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned. Directors, officers, employees or members of their families are prohibited from making purchases and/or sales of those securities in the open market or otherwise during the up-to-four-week interval from the time of the interview or article until after it publishes.